
On Tuesday May 23rd 2006 the American Chemical Society and the Canadian Society for Chemistry recognized the work of Professor Neil Bartlett as an International Historic Chemical Landmark. Professor Bartlett, working at UBC, demonstrated the first reaction of a noble gas by combining xenon with platinum hexafluoride.
The Experiment
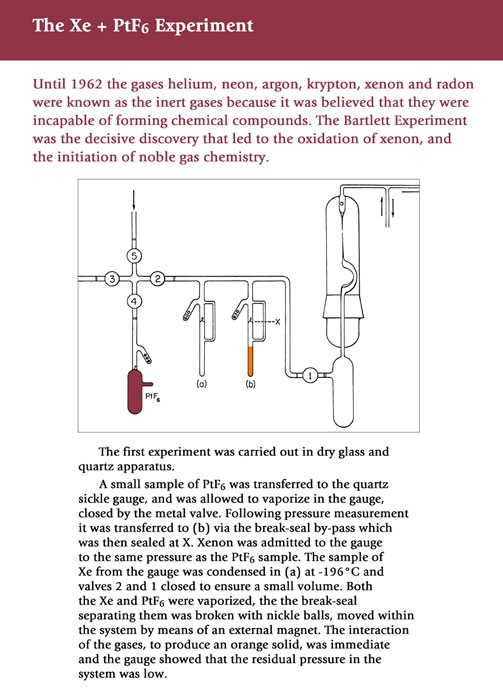
On the evening of March 23 1962, Neil Bartlett, working alone in the Chemistry Department at UBC, changed the face of chemistry. Until that day all chemistry textbooks were written with the fixed idea that the Group VIII elements He, Ne, Ar, Kr, Xe, and Rn, the Rare Gases, were chemically inert. In fact, they were often referred to as the Inert Gases. That led to the concept that atoms interact to achieve the 'desirable' state of a filled electron shell. In other words our discussion of valence was tied into the inertness of the filled electron shells of the Inert Gases.
Bartlett had previously shown that oxygen gas could be oxidized by platinum hexafluoride to afford O2PtF6 and had come to the realization that the “inertness” of the Rare Gases might be a consequence of the reagents employed rather than a law of nature. Consequently he mixed xenon gas with platinum hexafluoride and obtained a solid that had the formula XePtF6. This was formulated as a salt of Xe+ and PtF6- and the octet rule was no longer inviolable. The actual compound was later shown to be XeFPtF5 but the outcome was unchanged; all existing textbooks had to be rewritten and all subsequent ones had to adapt to this new reality.
Bartlett went on to develop this unprecedented chemistry at UBC and elsewhere: other individuals and groups have also contributed to the general research area which now comprises a substantial body of work, and it is almost commonplace to read about compounds of the rare gases. None the less it should always be remembered that the experimental verification of Bartlett’s seminal insight was carried out in the Chemistry Department of the UBC, as honoured in an International Historic Chemical Landmark.
Original Notes from the Bartlett Experiment, 23rd March, 1962.
