Abstract:
N-heterocyclic carbenes1 (NHCs) have become a hot topic in coordination and main group element chemistry, and the search for fine tuning via backbone substitution star-ted soon after the breakthrough discovery by Arduengo. Meanwhile, there have been several reports on synthesis and use of NHCs bearing one or two phosphorus-containing substituents in oxidation states I, III and V.2 After the initial synthesis of rigid tricyclic bis-NHCs by Bielawski3 and co-workers, a variety of bis- or multi-NHCs of different topology and rigidity were reported thus expanding the field of di- and multinuclear metal complexes. But the chemistry of redox-active NHCs still remained largely un-explored. Due to our first attempts to synthesize P-functional bis-NHCs,2b we were aware of this problem.
Recently, we described the synthesis of imidazole-2-thione-based 1,4-diphosphinines and started to explore the 1,4-diphosphinine chemistry.4 More recently, we synthesized tricyclic bis-NHCs with P-linkers in different oxidation states, i.e., P(V) I and P(III) II,5 and now striving for low-coordinate P(III) III (Fig. 1).
Furthermore, bis-NHC metal complexes of I and II will be presented, but also evidence for anionic bis-NHCs.
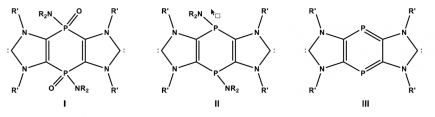
Figure 1. Variants of P-linked bis-NHCs.
1. Arduengo III, A. J.; Harlow, R. L.; Kline, M. J. Am. Chem. Soc. 1991, 113, 361- 363.
2. a) Bates, J. I.; Kennepohl, P.; Gates, D. P. Angew. Chem. Int. Ed. 2009, 48, 9844- 9847; b) Majhi, P. K.; Schnakenburg, G.; Kelemen, Z.; Nyulaszi, L.; Gates, D. P.; Streubel, R. Angew. Chem. Int. Ed. 2013, 52, 10080-10083.
3. Khramov, D. M.; Boydston, A. J.; Bielawski, C. W. Angew. Chem. Int. Ed. 2006, 45, 6186-6189.
4. a) Koner, A.; Kelemen, Z.; Schnakenburg, G.; Nyulászi, L.; Sasamori, T.; Streubel, R. Angew. Chem. Int. Ed. 2017, 56, 9231-9235; b) Koner, A.; Kelemen, Z.; Schna-kenburg, G.; Nyulászi, L.; Streubel, R. Chem. Commun. 2018, 54, 1182-1184.
5. Nauf Raz, N.; Schnakenburg, G.; Kelemen, Z.; Schnakenburg, G.; Nyulászi, L.; Streubel, R. 2019, submitted.
