
Nadine Borduas-Dedekind
Profile
Research and Teaching Interests
Atmospheric Chemistry
The atmosphere is like a large round bottom flask, where countless chemical reactions are taking place. For example, the emissions from anthropogenic and biogenic sources are the starting materials. The wind and turbulence act as stir bars and the sunlight acts as a hot plate. The round bottom flask also contains water, a crucial molecule to atmospheric chemistry, because it transports heat through the atmosphere, serves as a solvent for atmospheric aqueous reactions, and cleans the atmosphere through wet deposition. Water is also a precursor to the major oxidant in the atmosphere: the hydroxyl (OH) radical, the so-called atmospheric detergent. The real atmosphere has all these processes occurring simultaneously and we arguably understand a fraction of the chemical processes that lead to air pollution and climate change!
The NBD research group has an atmospheric organic chemistry expertise applied to the fields of indoor and outdoor air chemistry, and to biogeochemistry and atmospheric ice nucleation (Figure 1). Our research is collaborative to allow us to ask big-picture questions with implications for air quality and climate.
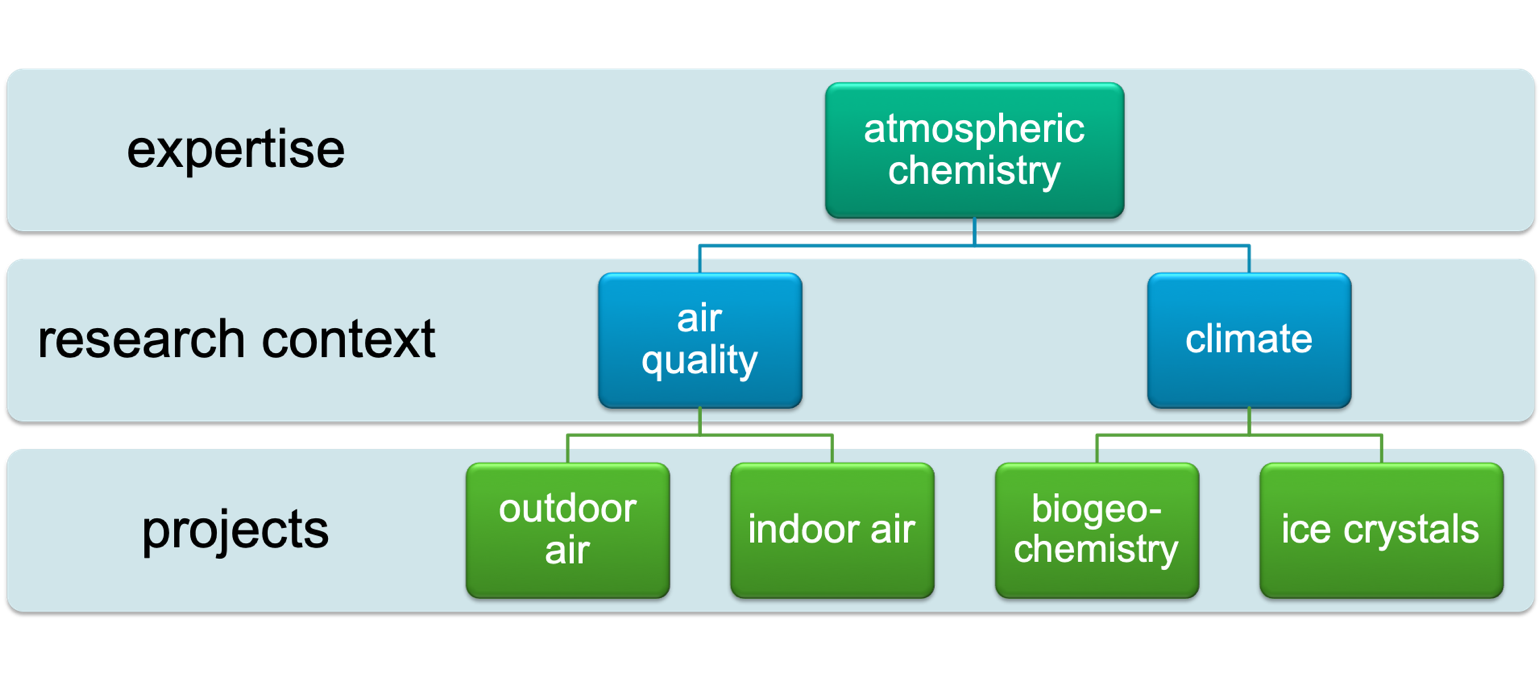
Figure 1: This hierarchy of research interests in the NBD Group includes applying atmospheric organic chemistry expertise to big picture problems such as air quality and climate change. The specific research projects fall within the context of indoor air, outdoor air, biogeochemistry and atmospheric ice nucleation.
Gas-Phase Chemical Kinetics and Mechanisms
The NBD Group is interested in studying the chemical mechanisms in which organic compounds, often pollutants, are oxidized and degraded in the atmosphere. Whether they are emitted volatile organic compounds (VOCs) from personal care products (fragrances), from boreal forests, (sesquiterpenes) or from the marine biota (organo-sulfur and -selenium), we want to fundamentally understand what happens to these molecules in the air. Four types of questions guide our research.
(1) How fast do these molecules get oxidized?
(2) What products do they generate and are these products of concern for human health?
(3) What are the mechanisms of oxidation of these molecules in our atmosphere?
(4) How are these molecules transformed in the atmosphere, by which oxidant and why?
We aim to provide a comprehensive assessment of the chemical reactions governing atmospheric processing, a goal only possible with state-of-the-art and sophisticated analytical chemistry tools and instruments. One instrument that effectively tackles all four of the key questions above is an online proton-transfer-reaction time-of-flight mass spectrometer (PTR-ToF-MS from Ionicon). Many of these experiments described will be conducted in a home-built Teflon bag smog chamber and in glass flow tubes. The PTR-ToF-MS will then measure the molecules of interest and follow their fate as a function of exposure to atmospheric processing (like photochemistry, oxidation, humidity, etc.).
Atmospheric Ice Nucleation
Cloud glaciation is an atmospheric process with important implications for climate and weather. Indeed, clouds made of liquid water and of ice crystals impact the global radiative balance of the atmosphere by reflecting incoming solar radiation and by absorbing outgoing terrestrial radiation. Ice nucleation is the process of liquid water or water vapor freezing. It occurs when supercooled water droplets freeze into ice crystals; a process which occurs spontaneously at or below – 38 °C and which is termed homogeneous freezing. Yet, ice exists at warmer temperatures due to heterogeneous freezing. Heterogeneous freezing requires an ice nucleating particle (INP) for water to condense on and form an ice crystal. The most relevant freezing pathway observed and modelled in mixed-phase clouds is immersion freezing which involves an INP submerged within a supercooled droplet. This freezing mechanism has been quantified to account for up to 70% of freezing in mixed phase clouds and is thus the focus of our droplet Freezing Ice Nuclei Counter (FINC) home-built instrument.
The NBD Group studies the ability of macromolecules, from organic aerosols and present in supercooled droplets, to nucleate ice in the atmosphere. We have two main research questions:
(1) How does organic matter affect the supercooled liquid to ice ratio in mixed-phase clouds?
(2) Can the chemical properties of organic aerosols be used to predict cloud glaciation temperatures to improve parameterization of cloud microphysics in models?
Contact
Curriculum Vitae
- Assistant Professor, University of British Columbia, Vancouver (Starting January 2021. For more information, visit this page.)
- SNSF Ambizione Fellow, ETH Zurich, Switzerland (2018-2021)
- NSERC Postdoctoral Fellow, ETH Zurich, Switzerland (2016-2018)
- Ph.D., Vanier Scholar, University of Toronto, Ontario (2011-2015)
- M.Sc., NSERC Julie-Payette, University of Toronto, Ontario (2009-2011)
- B.Sc., Université de Sherbrooke, Quebec (2005-2008)
- D.C.S., John Abbott College, Quebec (2003-2005)
Publications:
2019
Brennan, K. P., David, R. O., Borduas-Dedekind, N.* (2019) Ice nucleating particles measured in Swiss alpine snow samples are spatially, temporally and chemically heterogeneous. Atmospheric Chemistry and Physics Discussions, https://doi.org/10.5194/acp-2019-627
David, R. O.*, Cascajo, M. C., Brennan, K. P., Rösch, M., Els, N., Werz, J., Weichlinger, V., Boynton, L., Bogler, S., Borduas-Dedekind, N., Marcolli, C., Kanji, Z. A.* (2019) Development of the DRoplet Ice Nuclei Counter Zurich (DRINCZ): Validation and application to field collected snow samples, Atmospheric Measurement Techniques Discussion, https://doi.org/10.5194/amt-2019-213
Manfrin, A., Nizkorodov, S., Malecha, K., Getzinger, G., McNeill, K., Borduas-Dedekind, N.* (2019) Reactive oxygen species production from secondary organic aerosols: The importance of singlet oxygen. Environmental Science & Technology, 53: 8553-8562.
Waked, A. E., Demmans, K. Z., Hems, R. F., Reyes, L. M., Mallov, I., Daley, E., Hoch, L.B., Mastronardi, M. L., De La Franier, B. J., Borduas, N., Dicks, A. P.* (2019) The Green Chemistry Initiative’s Contributions to Chemistry Education at the University of Toronto and Beyond. Green Chemistry Letters & Reviews, 12: 187-195 – part of a special issue “Advances in Green Chemistry Education”
Borduas-Dedekind, N.*, Ossola, R., David, R. O., Boynton, L., Weichlinger, V., McNeill, K., Kanji, Z. A. (2019) Photomineralization mechanism changes the ability of dissolved organic matter to activate cloud droplets and to nucleate ice crystals. Atmospheric Chemistry and Physics Discussions, https://doi.org/10.5194/acp-2019-427, in review
Hems, R., Wang, C., Collins, D. B., Zhou, S., Borduas-Dedekind, N., Siegel, J. A., Abbatt, J. P. D. (2019) Sources of isocyanic acid (HNCO) indoors: a focus on cigarette smoke. Environmental Science: Processes & Impacts, 21:1334-1341
Leslie, M., Ridoli, M., Murphy, J. G., Borduas-Dedekind, N.* (2019) Isocyanic acid (HNCO) and its fate in the atmosphere: A review. Environmental Science: Processes & Impacts, 21:793-808
Manfrin, A., Borduas-Dedekind, N., Lau, K., McNeill, K.* (2019) Singlet oxygen photooxidation of peptidic oxazoles and thiazoles. Journal of Organic Chemistry, 84: 2439-2447
2018
Obhi, N. K., Mallov, I., Borduas, N., Rousseaux, S. A. L.,* Dicks, A. P.* (2018) Comparing industrial amination reactions in a combined class and laboratory green chemistry assignment. Journal of Chemical Education, 96: 93-99
Laban, T. L., van Zyl, P. G.*, Beukes, J. P., Vakkari, V., Jaars, K., Borduas-Dedekind, N., Josipovic, M., Thompson, A. M., Kulmala, M., Laakso, L. (2018) Seasonal influences on surface ozone variability in continental South Africa and implications for air quality. Atmospheric Chemistry and Physics, 18: 15491-15514
Wang, C., Collins, D. B., Hems, R. F., Borduas, N., Antinolo, M., Abbatt, J. P. D.* (2018) Exploring conditions for ultrafine particle formation from oxidation of cigarette smoke in indoor environments. Environmental Science & Technology. 52: 4623-4631
2016
Borduas, N.*, Abbatt, J. P. D., Murphy, J. G., So, S., da Silva, G. (2016) Gas-phase mechanisms of the reactions of reduced organic nitrogen compounds with OH radicals. Environmental Science & Technology. 50: 11723-11734
Borduas, N.*, Murphy, J. G., Wang, C., da Silva, G., Abbatt, J. P. D. (2016) Oxidation of nicotine by OH radicals: Kinetics, mechanisms and formation of HNCO. Environmental Science & Technology Letters. 3: 327-331
Borduas, N., Place, B., Wentworth, G. R., Abbatt, J. P. D., Murphy, J. G.* (2016) Solubility and reactivity of HNCO in water: Insights into HNCO’s fate in the atmosphere. Atmospheric Chemistry and Physics. 16 (2): 703-714
Borduas, N., Garland, R., Naidoo, M., (2016) Understanding ground level ozone pollution in the City of Johannesburg from 2004-2011. National Association for Clean Air, Nelspruit, Mpumalanga, Afrique de Sud – open access: http://www.naca.org.za/uploads/NACAconference_proceedings2016.pdf
2015
Borduas, N., da Silva, G., Murphy, J. G.*, Abbatt, J. P. D.* (2015) Experimental and theoretical understanding of the gas phase oxidation of atmospheric amides with OH radicals: Kinetics, products, and mechanisms. Journal of Physical Chemistry A. 119 (19): 4298-4308
2014
Ab Rani, M. A., Borduas, N., Colquhoun, V., Hanley, R., Johnson, H., Larger, S., Lickiss, P. D.*, Llopis-Mestre, V., Luu, S., Mogstad, M., Oczipka, P., Sherwood, J. R., Welton, T.*, Xing, J.-Y. (2014) The potential of methylsiloxanes as solvents for synthetic chemistry applications. Green Chemistry. 16 (3): 1282-1296
2013
Borduas, N., Abbatt, J. P. D.*, Murphy, J. G.* (2013) Gas phase oxidation of monoethanolamine (MEA) with OH radicals and ozone: Kinetics, products and particles. Environmental Science & Technology. 47 (12): 6377−6383
2012
VandenBoer, T. C., Markovic, M. Z., Petroff, A., Czar, M. F., Borduas, N., Murphy, J. G.* (2012) Ion chromatographic separation and quantitation of alkyl methylamines and ethylamines in atmospheric gas and particulate matter using preconcentration and suppressed conductivity detection. Journal of Chromatography A. 1252: 74-83
2011
Borduas, N., Lough, A. S., Dong, V. M.* (2011) Cyclopalladation of phenylbenzamides: Synthesis and structure of bimetallic palladium(II)-complexes. Inorganica Chemica Acta. 369 (1): 247-252
2010
Yeung, C. S., Zhao, X., Borduas, N., Dong, V. M.* (2010) Pd-Catalyzed ortho-arylation of phenylacetamides, benzamides, and anilides with simple arenes using sodium persulfate. Chemical Science. 1: 331-336
Baslé, O., Borduas, N., Dubois, P., Chapuzet, J. M., Chan, T.-H., Lessard, J.*, Li, C.-J.* (2010) Aerobic and electrochemical oxidative cross-dehydrogenative-coupling (CDC) reaction in an imidazolium-based ionic liquid. Chemistry, A European Journal. 16 (27): 8162-8166
2008
Borduas, N., Powell, D. A.* (2008) Copper-catalyzed oxidative coupling of benzylic C−H bonds with 1,3-dicarbonyl compounds. Journal of Organic Chemistry. 73 (19): 7822–7825
