Abstract:
Traditionally, strategies for the design of homogeneous catalysts utilize metals as the locus of all reactivity. Recently, novel strategies have been introduced that enable metal-ligand bifunctional catalysis and ‘ligand redox catalysis’. The former involves direct participation of the ligand in bond-breaking and –making processes during substrate activation and turnover (‘reactive ligands’). The latter mainly focusses on strategies where the ligand displays redox-chemistry infers reactivity at the metal center or at the metal-bound substrate (‘redox-active ligands’). Often, this ligand-based reactivity is embedded within the rigid tridentate coordination offered by a pincer platform.
We have introduced novel reactive and/or redox-active tridentate ligand designs that show fascinating reactivity in both mono- and multinuclear base and noble metal complexes. The potential of this approach will be sketched using selected examples on formic acid dehydrogenation, CO cleavage, C-H amination, homolytic bond activation, electrocatalytic C-X activation and azide activation by virtue of the reactive and redox-active ligand platforms.
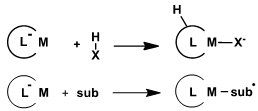
Generic metal-ligand bifunctional substrate activation (top) and ligand redox-activity (bottom)
Acknowledgements
This research is funded by the ERC, NWO and the University of Amsterdam.
Recent Literature from the group:
J. Am. Chem. Soc. 2014, 136, 11574; Chem. Eur. J. 2015, 21, 7297; Angew. Chem. Int. Ed. 2015, 54, 1516; Chem. Eur. J. 2015, 21, 12683; Catal. Sci. Technol. 2016, 6, 1320; Dalton. Trans. 2016, 45, 10989; Angew. Chem. Int. Ed. 2016, 55, 2406; Angew. Chem. Int. Ed. 2016, 55, 8381; Angew. Chem. Int. Ed. 2016, 55, 10042; Chem. Eur. J. 2016, 22, 13965; Organometallics 2017, 36, 1541; J. Am. Chem. Soc. 2017, 139, 5117 ; Chem. Eur. J. 2017, 23, 5585; Chem. Eur. J. 2017, 23, 12709; Chem. Eur. J. 2019, 25, 2651.
