
Glenn Sammis
Profile
Research and Teaching Interests
Our research focuses on the development of new methods for the syntheses of architecturally complex natural products. This encompasses the development of new methods for the fluorination of alkyl and aryl radicals, the use of oxygen- and nitrogen-centered radicals for the synthesis of heterocycles, organometallic reagents for the development of new bond construction techniques, the enantioselective synthesis of building blocks for total synthesis, and the development and evaluation of proposed biogenetic pathways through biomimetic synthesis. Central to efficient methods development is a fundamental understanding of reactivity and reaction mechanisms.
Recent highlights of our research are:
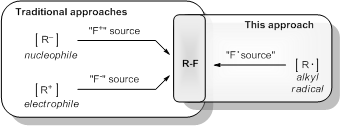
- Fluorine Atom Transfer to Alkyl Radicals. The development of new synthetic technologies for the selective fluorination of organic compounds has increased with the escalating importance of fluorine-containing pharmaceuticals. Traditional methods potentially applicable to drug synthesis rely on the use of ionic forms of fluorine (F– or F+). Radical methods, while potentially attractive as a complementary approach, are hindered by a paucity of safe sources of atomic fluorine (F·). We have developed a new approach to alkyl fluorination that utilizes the reagent N-fluorobenzenesulfonimide as a fluorine transfer agent to alkyl radicals (J. Am. Chem. Soc. 2012, 134, 4026-4029). This approach is successful for a broad range of alkyl radicals, including primary, secondary, tertiary, benzylic, and heteroatom stabilized radicals. Furthermore, calculations reveal that Selectfluor®, and related ionic reagents, are likely candidates for further expansion of this approach to polar reaction media. The use of these reagents in alkyl radical fluorination has the potential to enable powerful new transformations that otherwise would take multiple synthetic steps.
- Radical Relay Cyclizations. We have developed an efficient method for the rapid construction of bioactive carbo- and heterocycles using radical relay cyclization initiated by alkoxy radicals. This new method allows for the rapid increase in molecular complexity from simple starting materials though the conversion of unactivated C-H bonds into reactive intermediates. The cyclizations are effective for the synthesis of a wide range of cyclopentane, pyrrolidine, tetrahydropyran, and tetrahydrofurans derivatives.
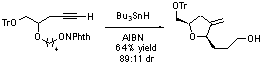
We are currently further exploring this new synthetic method in the total synthesis of both (–)-lepadiformine A and (–)-amphidinolide K. Each synthesis involves synthesizing complex heterocyclic fragments by using sequential or cascade reactions in which complexity is rapidly achieved from simple linear precursors. We are also currently developing new, non-radical synthetic methods for the syntheses of heterocycles.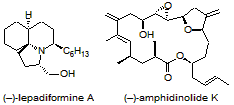
Contact
Curriculum Vitae
B.S. (honors), Stanford University (1999); Ph.D., Harvard University (Eric. N. Jacobsen, 2004); National Institutes of Health Postdoctoral Fellow, Princeton University (Erik. J. Sorensen, 2004-2006)
