
Marco Ciufolini
Profile
Research and Teaching Interests
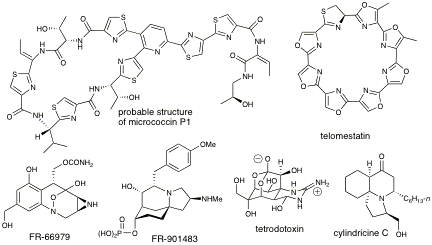
Research in this group is directed toward the total synthesis of nitrogenous natural products that possess interesting structures and useful biological activities. In the course of these endeavors, great emphasis is placed on the development of innovative, efficient, and environmentally responsible preparative reactions. Possibilities are currently being sought in areas as diverse as main-group, transition-, and f-block organometallic chemistry; enzymatic transformations; photochemistry; heterocycles; hypervalent iodine chemistry, etc. Problems of current interest encompass the synthesis of thiopeptide antibiotics (genetic modulators and inhibitors of protein synthesis); of polyoxazole natural products, e.g. telomestatin (potent and selective inhibitor of human telomerase); of substances incorporating medium-ring nitrogen heterocycles or surrogates thereof; e.g. mitomycinoids (antitumor agents); and of spirocyclic alkaloids (immunusuppressants; cytotoxic agents; ion-channel blockers; and so on). These substances are of great interest in oncology, neurology, microbiology, immunology, and genetics. Our research is thus especially relevant to the objectives of UBC's Drug Research Institute, an exciting multidisciplinary initiative, and of the pharmaceutical industry.
Several compounds of current interest in this group are shown below.

Contact
Curriculum Vitae
B.S. (chemistry) 1978, Spring Hill College, Mobile AL; Ph.D. 1981, University of Michigan, Ann Arbor MI (M. Koreeda); Postdoc 1982-84, Yale University, New Haven, CT (S. Danishefsky). A. P. Sloan Fellow, 1994-98; Merck & Co. Academic Development Prize, 2001-2. Canada Research Chair in Synthetic Organic Chemistry, 2004
